 All Categories
All Categories
 Intermediate
Intermediate
 400-780-8018
400-780-8018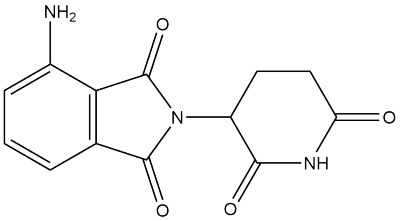

 Favorites
Favorites
 Share
Share Product Information
Product Information Product Description
Product DescriptionPomalidomide is a promising immunomodulatory drug (IMiD) with significant activity in multiple myeloma (MM). Pomalidomide acts an E3 ligase recruiter exploited by PROTACs to degrade target proteins. Pomalidomide also works as a molecular glue that can simultaneously bind to the CRBN.
Pomalidomide is an FDA-approved drug for the treatment of multiple myeloma based on its binding to an E3 ligase component cereblon (CRBN).
In vitro studies[2] (for reference only)
Pomalidomide has direct anti-proliferative (by up-regulation the expression of p21 WAF1 tumor suppressor gene) and pro-apoptotic effects (by enhancing MM sensitivity to Fas-induced and TRAIL/Apo2L-induced apoptosis via a caspase-8-dependent mechanism)[2].
OPM2 and RPMI8226 cell lines were cultured at 24h and 48h and incubated with Pomalidomide (from 0.01 μM to 50 μM). Pomalidomide significantly suppressed cell proliferation of RPMI8226 and OPM2 cells at 48h with IC50 values of 8 μM and 10 μM, respectively.
The apoptotic effect of Pomalidomide was evaluated on MM cell lines and patients' MM cells by flow cytometry. MM cell lines were cultured with Pomalidomide (0.01-50 μM, at 24-72h). Pomalidomide significantly induced apoptosis cell death (P<0.05).
In vivo studies[3] (for reference only)
Traumatic brain injury (TBI) usually causes emotional disturbances and cognitive dysfunction. Pomalidomide was evaluated in a rat model of moderate to severe TBI induced by controlled cortical impact. Post-TBI intravenous administration of Pomalidomide (0.5 mg/kg administration at 5 h) significantly decreased the TBI-induced injury lesion volume and functional impairments, neurodegeneration, neuronal apoptosis, and provided anti-inflammatory properties.
References:
[1] Arleigh R McCurdy 1, Martha Q Lacy. Pomalidomide and its clinical potential for relapsed or refractory multiple myeloma: an update for the hematologist. Ther Adv Hematol. 2013 Jun;4(3):211-6. doi: 10.1177/2040620713480155. https://pubmed.ncbi.nlm.nih.gov/23730498/
[2] Tommasina Guglielmelli, et al. mTOR pathway activation in multiple myeloma cell lines and primary tumour cells: pomalidomide enhances cytoplasmic-nuclear shuttling of mTOR protein. Oncoscience. 2015 Apr 6;2(4):382-94. doi: 10.18632/oncoscience.148. eCollection 2015. https://pubmed.ncbi.nlm.nih.gov/26097872/
[3] Jin-Ya Wang, et al. Pomalidomide mitigates neuronal loss, neuroinflammation, and behavioral impairments induced by traumatic brain injury in rat. J Neuroinflammation. 2016 Jun 28;13(1):168. doi: 10.1186/s12974-016-0631-6. https://pubmed.ncbi.nlm.nih.gov/27353053/

 Related Products
Related Products Browsing history
Browsing history Toolbox
Toolbox Contact Us
Contact Us




 Inquiry
Inquiry
 Fill in the custom synthesis service requirement form
Fill in the custom synthesis service requirement form
 Shanghai ICP Record Number 2023026221-1
Shanghai Public Network Security Registration No.31011502400732
Shanghai ICP Record Number 2023026221-1
Shanghai Public Network Security Registration No.31011502400732